Nanolute
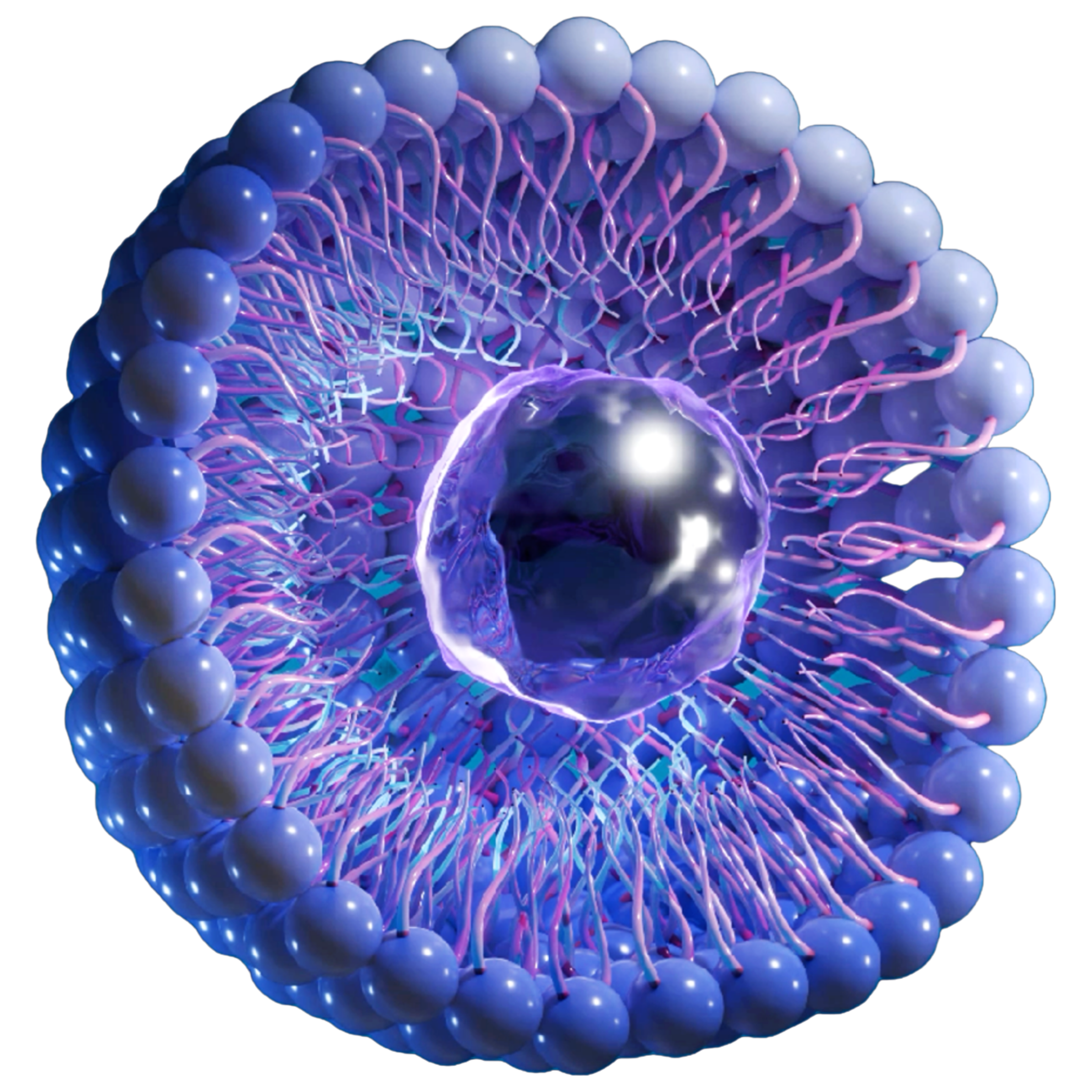
Technology

Advantages of Nanolute Technology
-
Facilitates better adhesion of
Sirolimus on the balloon surface -
Circumferential coating ensures
homogeneous drug delivery -
Effective drug transfer to the
deepest layer of the vessel -
Better in-tissue bioavailability of
Sirolimus
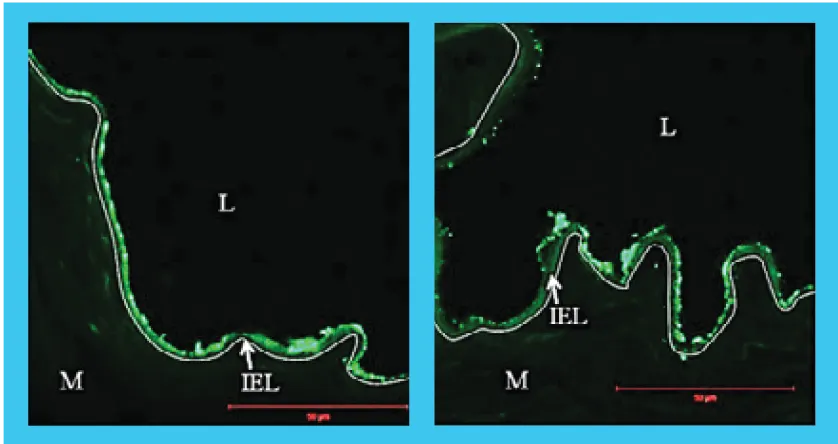
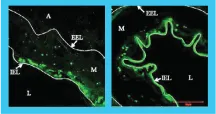
SIROLIMUS DISTRIBUTION STUDY
1 HR
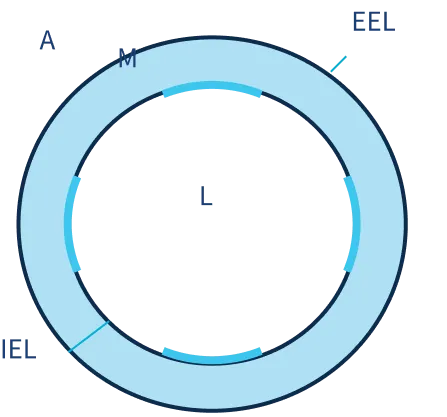
Intima
60-70% above IEL
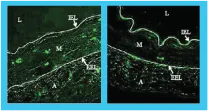
DAY 3
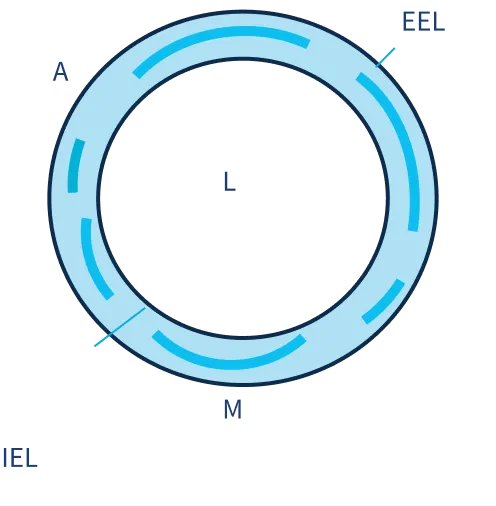
Media
30-40% within Media
DAY 7
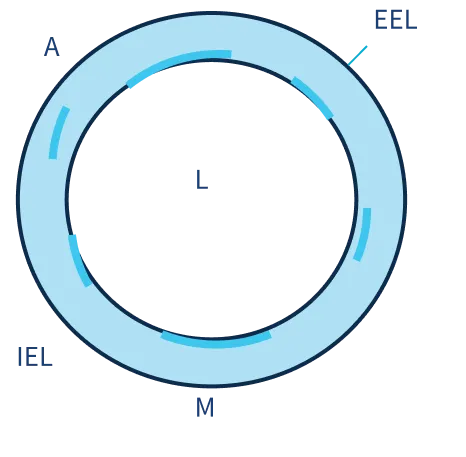
Adventitia
30-40% below IEL
Improves Lipophilicity of Sirolimus
- Lipophilicity is the ability of a drug to dissolve in fats, oils, and lipids.
- Sirolimus possesses poor lipophilicity and hence requires phospholipids.
- NANOLUTE technology delivers polymer-free nanocarriers containing sirolimus surrounded by an encapsulation of a proprietary drug carrier, i.e., a phospholipid, and improves lipophilicity.
Solving Paclitaxel’s shortcomings
- Lower Toxicity, Proven Safety
- Stable Coating & Minimal Shedding
- Less Embolization & Safer Procedures
- Optimized Drug Retention & Delivery

