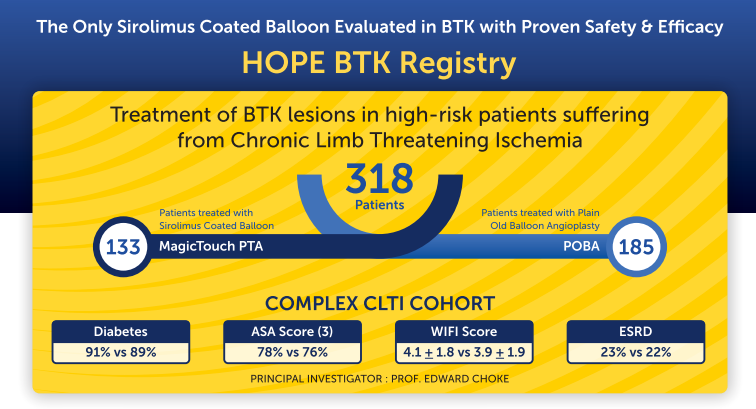
Charing Cross 2025 — The HOPE BTK Registry, presented by Prof. Edward Choke has reinforced the safety and efficacy of MagicTouch PTA (Concept Medical Inc.), the world’s only Sirolimus-Coated Balloon (SCB) evaluated against POBA for below-the-knee (BTK) lesions in patients with Chronic Limb Threatening Ischemia (CLTI), with safety and efficacy advantages at long term follow-up of 3 years.
The study included 318 high-risk patients, with 133 treated using MagicTouch PTA (Sirolimus-Coated Balloon) and 185 with plain old balloon angioplasty (POBA). Patient characteristics reflected a complex CLTI cohort, with high prevalence of comorbidities:
- Diabetes: 91% (Sirolimus Coated Balloon) vs 89% (POBA)
- ASA score (3): 78% (Sirolimus Coated Balloon) vs 76% (POBA)
- ESRD: 23% (Sirolimus Coated Balloon) vs 22% (POBA)
- WIFI score: 4.1 ± 1.8 (Sirolimus Coated Balloon) vs 3.9 ± 1.9 (POBA)
Key 3-Year Clinical Outcomes
- Freedom from Target Lesion Revascularization (TLR):
76.5% with Sirolimus Coated Balloon vs 64.9% with POBA
(HR 0.58; p=0.033) - Freedom from Major Amputation:
92.8% with Sirolimus Coated Balloon vs 85.7% with POBA
(HR 0.62; p=0.32) - Amputation-Free Survival (AFS):
60.9% with Sirolimus Coated Balloon vs 47.4% with POBA
(HR 0.66; p=0.014) - Overall Survival:
62.4% with Sirolimus Coated Balloon vs 53.9% with POBA
(HR 0.71; p=0.049)
Clinical Significance
These results demonstrate that MagicTouch PTA Sirolimus Coated Balloon was associated with sustained clinical benefits up to 36 months, reducing the need for repeat interventions while improving survival rates in a high-risk CLTI population.
Compared to POBA, the Sirolimus-Coated Balloon showed:
- Lower BTK re-intervention rates
- Better amputation-free survival
- Improved overall survival
Prof. Edward Choke, Principal Investigator, concluded that the HOPE BTK Registry strengthens the evidence base for Sirolimus-coated balloon therapy in BTK interventions, positioning MagicTouch PTA as a safe and effective option for patients with CLTI.
The HOPE BTK findings mark a significant step forward in peripheral vascular treatment, offering new hope for patients facing the challenges of CLTI.
