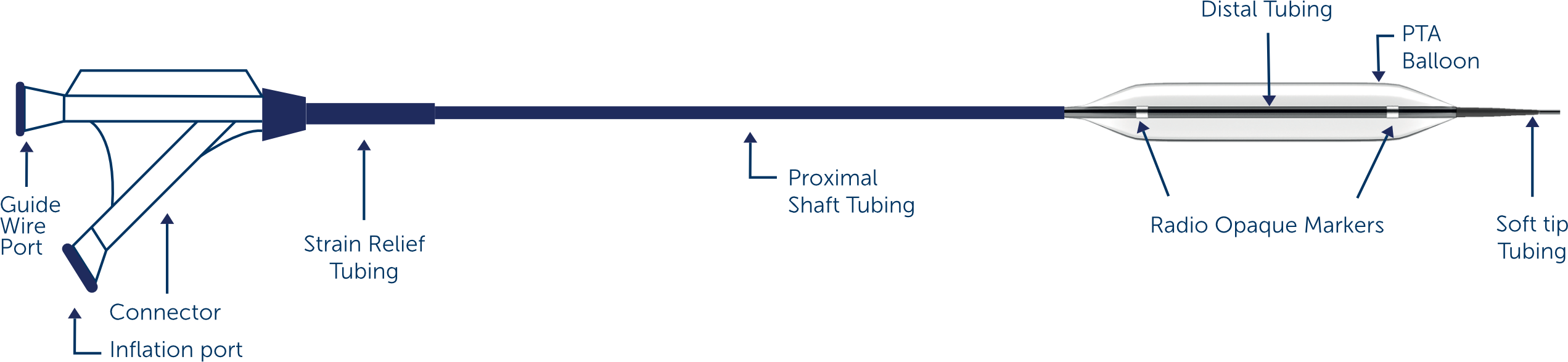
The Midas PTA Dilatation catheter is intended for percutaneous transluminal angioplasty in a wide array of peripheral arteries. One Point Solution PTA dilatation catheter for treating Peripheral Vascular disease including Iliac, Femoral, Popliteal, Tibial, Peroneal arteries and obstructive lesions of native or synthetic arteriovenous dialysis fistulae.


Wide Size Matrix
Wide Size matrix and Guide wire Compatibility to treat the majority of Peripheral Anatomies.

Crossablity
Designed for Excellent Pushability & Trackability

Low-Profile
5F Compatible Catheter with atraumatic Low profile Tip of 0.020” for better Crossability while treating the Above and BTK lesions

Re-wrap
Better Balloon Re‐wrapping for low crossing profile

Design
Optimized 2-lumen shaft construction
COATING
Circumferential Coating is
performed under Low Pressure inflation
leading to 100% surface area coating
REFOLDING
3 Folds
INFLATION
Homogeneous Drug Delivery
due to Circumferential Coating
TECHNOLOGY
An innovative proprietary Nanolute technology providing better bioavailability of SIROLIMUS
Sirolimus: A drug with proven safety profile
COATING
Unique coating technology leads to 100% balloon surface coating
A biocompatible phospholipid drug carrier improving the adhesion property of Sirolimus
03
Balloon
| Delivery System | Over-The-Wire |
| Balloon Compliance | Semi-Compliant |
| No. of Folds | 3 & 6 |
| Balloon Material | Polyamide |
| Guidewire Compatibility | 0.014″, 0.018″, 0.035″ |
DRUG/EXCIPIENT
Drug
Drug Dose
Drug Carrier
1.27µg/mm²
Phospholipid
Catheter design
Rapid Exchange(RX) Design
| (0.018” OTW) | Length (Shaft Length = 90 cm) | ||||||
|---|---|---|---|---|---|---|---|
| 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | |
| 2.00 | CMDW18001 | CMDW18002 | CMDW18003 | CMDW18004 | CMDW18005 | CMDW18006 | CMDW18007 |
| 2.50 | CMDW18008 | CMDW18009 | CMDW18010 | CMDW18011 | CMDW18012 | CMDW18013 | CMDW18014 |
| 3.00 | CMDW18015 | CMDW18016 | CMDW18017 | CMDW18018 | CMDW18019 | CMDW18020 | CMDW18021 |
| 3.50 | CMDW18022 | CMDW18023 | CMDW18024 | CMDW18025 | CMDW18026 | CMDW18027 | CMDW18028 |
| 4.00 | CMDW18029 | CMDW18030 | CMDW18031 | CMDW18032 | CMDW18033 | CMDW18034 | CMDW18035 |
| 5.00 | CMDW18036 | CMDW18037 | CMDW18038 | CMDW18039 | CMDW18040 | CMDW18041 | CMDW18042 |
| 5.50 | CMDW18043 | CMDW18044 | CMDW18045 | CMDW18046 | CMDW18047 | CMDW18048 | CMDW18049 |
| 6.00 | CMDW18050 | CMDW18051 | CMDW18052 | CMDW18053 | CMDW18054 | CMDW18055 | CMDW18056 |
| 7.00 | CMDW18057 | NA | NA | NA | NA | NA | NA |
| 0.018″ OTW | Length (Shaft Length = 120 cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| 2.00 | CMDW18058 | CMDW18059 | CMDW18060 | CMDW18061 | CMDW18062 | CMDW18063 | CMDW18064 | |
| 2.50 | CMDW18065 | CMDW18066 | CMDW18067 | CMDW18068 | CMDW18069 | CMDW18070 | CMDW18071 | |
| 3.00 | CMDW18072 | CMDW18073 | CMDW18074 | CMDW18075 | CMDW18076 | CMDW18077 | CMDW18078 | |
| 3.50 | CMDW18079 | CMDW18080 | CMDW18081 | CMDW18082 | CMDW18083 | CMDW18084 | CMDW18085 | |
| 4.00 | CMDW18086 | CMDW18087 | CMDW18088 | CMDW18089 | CMDW18090 | CMDW18091 | CMDW18092 | |
| 5.00 | CMDW18093 | CMDW18094 | CMDW18095 | CMDW18096 | CMDW18097 | CMDW18098 | CMDW18099 | |
| 5.50 | CMDW18100 | CMDW18101 | CMDW18102 | CMDW18103 | CMDW18104 | CMDW18105 | CMDW18106 | |
| 6.00 | CMDW18107 | CMDW18108 | CMDW18109 | CMDW18110 | CMDW18111 | CMDW18112 | CMDW18113 | |
| 7.00 | CMDW18114 | NA | NA | NA | NA | NA | NA | |
| 0.018″ OTW | Length (Shaft Length = 150 cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| 2.00 | CMDW18115 | CMDW18116 | CMDW18117 | CMDW18118 | CMDW18119 | CMDW18120 | CMDW18121 | |
| 2.50 | CMDW18122 | CMDW18123 | CMDW18124 | CMDW18125 | CMDW18126 | CMDW18127 | CMDW18128 | |
| 3.00 | CMDW18129 | CMDW18130 | CMDW18131 | CMDW18132 | CMDW18133 | CMDW18134 | CMDW18135 | |
| 3.50 | CMDW18136 | CMDW18137 | CMDW18138 | CMDW18139 | CMDW18140 | CMDW18141 | CMDW18142 | |
| 4.00 | CMDW18143 | CMDW18144 | CMDW18145 | CMDW18146 | CMDW18147 | CMDW18148 | CMDW18149 | |
| 5.00 | CMDW18150 | CMDW18151 | CMDW18152 | CMDW18153 | CMDW18154 | CMDW18155 | CMDW18156 | |
| 5.50 | CMDW18157 | CMDW18158 | CMDW18159 | CMDW18160 | CMDW18161 | CMDW18162 | CMDW18163 | |
| 6.00 | CMDW18164 | CMDW18165 | CMDW18166 | CMDW18167 | CMDW18168 | CMDW18169 | CMDW18170 | |
| 7.00 | CMDW18171 | NA | NA | NA | NA | NA | NA | |
| 0.014″ OTW | Length (Shaft Length = 90 cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| 1.50 | CMDW14001 | NA | NA | NA | NA | NA | NA | NA | |
| 2.00 | NA | CMDW14002 | CMDW14003 | CMDW14004 | CMDW14005 | CMDW14006 | CMDW14007 | CMDW14008 | |
| 2.50 | NA | CMDW14009 | CMDW14010 | CMDW14011 | CMDW14012 | CMDW14013 | CMDW14014 | CMDW14015 | |
| 3.00 | NA | CMDW14016 | CMDW14017 | CMDW14018 | CMDW14019 | CMDW14020 | CMDW14021 | CMDW14022 | |
| 3.50 | NA | CMDW14023 | CMDW14024 | CMDW14025 | CMDW14026 | CMDW14027 | CMDW14028 | CMDW14029 | |
| 4.00 | NA | CMDW14030 | CMDW14031 | CMDW14032 | CMDW14033 | CMDW14034 | CMDW14035 | CMDW14036 | |
| 0.014″ OTW | Length (Shaft Length = 120 cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| 1.50 | CMDW14037 | NA | NA | NA | NA | NA | NA | NA | |
| 2.00 | NA | CMDW14038 | CMDW14039 | CMDW14040 | CMDW14041 | CMDW14042 | CMDW14043 | CMDW14044 | |
| 2.50 | NA | CMDW14045 | CMDW14046 | CMDW14047 | CMDW14048 | CMDW14049 | CMDW14050 | CMDW14051 | |
| 3.00 | NA | CMDW14052 | CMDW14053 | CMDW14054 | CMDW14055 | CMDW14056 | CMDW14057 | CMDW14058 | |
| 3.50 | NA | CMDW14059 | CMDW14060 | CMDW14061 | CMDW14062 | CMDW14063 | CMDW14064 | CMDW14065 | |
| 4.00 | NA | CMDW14066 | CMDW14067 | CMDW14068 | CMDW14069 | CMDW14070 | CMDW14071 | CMDW14072 | |
| 0.014″ OTW | Length (Shaft Length = 150 cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| 1.50 | CMDW14073 | NA | NA | NA | NA | NA | NA | NA | |
| 2.00 | NA | CMDW14074 | CMDW14075 | CMDW14076 | CMDW14077 | CMDW14078 | CMDW14079 | CMDW14080 | |
| 2.50 | NA | CMDW14081 | CMDW14082 | CMDW14083 | CMDW14084 | CMDW14085 | CMDW14086 | CMDW14087 | |
| 3.00 | NA | CMDW14088 | CMDW14089 | CMDW14090 | CMDW14091 | CMDW14092 | CMDW14093 | CMDW14094 | |
| 3.50 | NA | CMDW14095 | CMDW14096 | CMDW14097 | CMDW14098 | CMDW14099 | CMDW14100 | CMDW14101 | |
| 4.00 | NA | CMDW14102 | CMDW14103 | CMDW14104 | CMDW14105 | CMDW14106 | CMDW14107 | CMDW14108 | |
| 0.035″ OTW | Length (Shaft Length = 90 cm = 900 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| Diameter | 3.00 | CMDW35057 | CMDW35058 | CMDW35059 | CMDW35060 | NA | NA | NA | NA |
| 4.00 | CMDW35061 | CMDW35062 | CMDW35063 | CMDW35064 | CMDW35065 | CMDW35066 | CMDW35067 | CMDW35068 | |
| 5.00 | CMDW35069 | CMDW35070 | CMDW35071 | CMDW35072 | CMDW35073 | CMDW35074 | CMDW35075 | CMDW35076 | |
| 6.00 | CMDW35077 | CMDW35078 | CMDW35079 | CMDW35080 | CMDW35081 | CMDW35082 | CMDW35083 | CMDW35084 | |
| 7.00 | CMDW35085 | CMDW35086 | CMDW35087 | CMDW35088 | CMDW35089 | CMDW35090 | CMDW35091 | CMDW35092 | |
| 8.00 | CMDW35093 | CMDW35094 | CMDW35095 | CMDW35096 | CMDW35097 | CMDW35098 | CMDW35099 | CMDW35100 | |
| 9.00 | CMDW35101 | CMDW35102 | CMDW35103 | CMDW35104 | NA | NA | NA | NA | |
| 10.00 | CMDW35105 | CMDW35106 | CMDW35107 | CMDW35108 | NA | NA | NA | NA | |
| 12.00 | CMDW35109 | CMDW35110 | CMDW35111 | CMDW35112 | NA | NA | NA | NA | |
| 0.035″ OTW | Length (Shaft Length = 45 cm = 450 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| Diameter | 3.00 | CMDW35113 | CMDW35114 | CMDW35115 | CMDW35116 | NA | NA | NA | NA |
| 4.00 | CMDW35117 | CMDW35118 | CMDW35119 | CMDW35120 | CMDW35121 | CMDW35122 | NA | NA | |
| 5.00 | CMDW35123 | CMDW35124 | CMDW35125 | CMDW35126 | CMDW35127 | CMDW35128 | NA | NA | |
| 6.00 | CMDW35129 | CMDW35130 | CMDW35131 | CMDW35132 | CMDW35133 | CMDW35134 | NA | NA | |
| 7.00 | CMDW35135 | CMDW35136 | CMDW35137 | CMDW35138 | CMDW35139 | NA | NA | NA | |
| 8.00 | CMDW35140 | CMDW35141 | CMDW35142 | CMDW35143 | CMDW35144 | NA | NA | NA | |
| 9.00 | CMDW35145 | CMDW35146 | CMDW35147 | CMDW35148 | NA | NA | NA | NA | |
| 10.00 | CMDW35149 | CMDW35150 | CMDW35151 | CMDW35152 | NA | NA | NA | NA | |
| 0.035″ OTW | Length (Shaft Length = 130 cm = 1300 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| Diameter | 3.00 | CMDW35153 | CMDW35154 | CMDW35155 | CMDW35156 | NA | NA | NA | NA |
| 4.00 | CMDW35157 | CMDW35158 | CMDW35159 | CMDW35160 | CMDW35161 | CMDW35162 | CMDW35163 | CMDW35164 | |
| 5.00 | CMDW35165 | CMDW35166 | CMDW35167 | CMDW35168 | CMDW35169 | CMDW35170 | CMDW35171 | CMDW35172 | |
| 6.00 | CMDW35173 | CMDW35174 | CMDW35175 | CMDW35176 | CMDW35177 | CMDW35178 | CMDW35179 | CMDW35180 | |
| 7.00 | CMDW35181 | CMDW35182 | CMDW35183 | CMDW35184 | CMDW35185 | CMDW35186 | CMDW35187 | CMDW35188 | |
| 8.00 | CMDW35189 | CMDW35190 | CMDW35191 | CMDW35192 | CMDW35193 | CMDW35194 | CMDW35195 | CMDW35196 | |
| 9.00 | CMDW35197 | CMDW35198 | CMDW35199 | CMDW35200 | NA | NA | NA | NA | |
| 10.00 | CMDW35201 | CMDW35202 | CMDW35203 | CMDW35204 | NA | NA | NA | NA | |
| 12.00 | CMDW35205 | CMDW35206 | CMDW35207 | CMDW35208 | NA | NA | NA | NA | |
| 0.035″ OTW | Length (Shaft Length = 150 cm = 1500 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20.00 | 40.00 | 60.00 | 80.00 | 100.00 | 120.00 | 150.00 | 200.00 | ||
| Diameter | 3.00 | CMDW35209 | CMDW35210 | CMDW35211 | CMDW35212 | NA | NA | NA | NA |
| 4.00 | CMDW35213 | CMDW35214 | CMDW35215 | CMDW35216 | CMDW35217 | CMDW35218 | CMDW35219 | CMDW35220 | |
| 5.00 | CMDW35221 | CMDW35222 | CMDW35223 | CMDW35224 | CMDW35225 | CMDW35226 | CMDW35227 | CMDW35228 | |
| 6.00 | CMDW35229 | CMDW35230 | CMDW35231 | CMDW35232 | CMDW35233 | CMDW35234 | CMDW35235 | CMDW35236 | |
| 7.00 | CMDW35237 | CMDW35238 | CMDW35239 | CMDW35240 | CMDW35241 | CMDW35242 | CMDW35243 | CMDW35244 | |
| 8.00 | CMDW35245 | CMDW35246 | CMDW35247 | CMDW35248 | CMDW35249 | CMDW35250 | CMDW35251 | CMDW35252 | |
| 9.00 | CMDW35253 | CMDW35254 | CMDW35255 | CMDW35256 | NA | NA | NA | NA | |
| 10.00 | CMDW35257 | CMDW35258 | CMDW35259 | CMDW35260 | NA | NA | NA | NA | |
| 12.00 | CMDW35261 | CMDW35262 | CMDW35263 | CMDW35264 | NA | NA | NA | NA | |
| Guiding Catheter Compatibility | |
|---|---|
| 0.014” | 5F |
| 0.018” | 5F, 6F and 7F |
| 0.035” | 7F, 8F and 9F |
| Sheath Compatibility | |
|---|---|
| 0.014” | 4F |
| 0.018” | 4F to 5F |
| 0.035” | 5F to 7F |

