ABLUMINUS DES+ designed by propriety ENVISOLUTION technology in a way that address the unmet clinical needs specially in Diabetes Mellitus and Acute Myocardial infarction patients with Coronary Artery Disease.

Designed to treat patients with Diabetes Mellitus and Acute Myocardial Infarction, leveraging Envisolution technology, it features fusion coating with biodegradable film and edge coating to ensure homogeneous drug distribution and healing while effective treatment of diffused restenosis.
Only approved Sirolimus DES+ for DM & AMI Treatment
Studied in World’s Largest RCT for DM
CE Approved
02
Key Indications:
- Diabetes Mellitus
- Acute Myocardial Infarction
- Symptomatic CAD (ACS)
03
Envisolution is an innovative drug delivery platform designed for cardiovascular interventions. It features a homogeneous, biodegradable polymer-based matrix with an abluminal coating that incorporates the drug on both the stent and the exposed parts of the balloon. This design allows for the uniform delivery of the drug to the target site, promoting sustained therapeutic effects.
Key Features
Biodegradable Film
The formation of circular film with biodegradable polymer facilitate maximum drug delivery in blood wet conditions
Abluminal Coating
Facilitate mono directional drug release and less systemic exposure of drug leading to faster re-endothelistation
Fusion Coating
Coating on the stent and exposed parts of the balloon facilitates homogeneous drug delivery, which addresses diffused proliferative disease and focal restenosis.
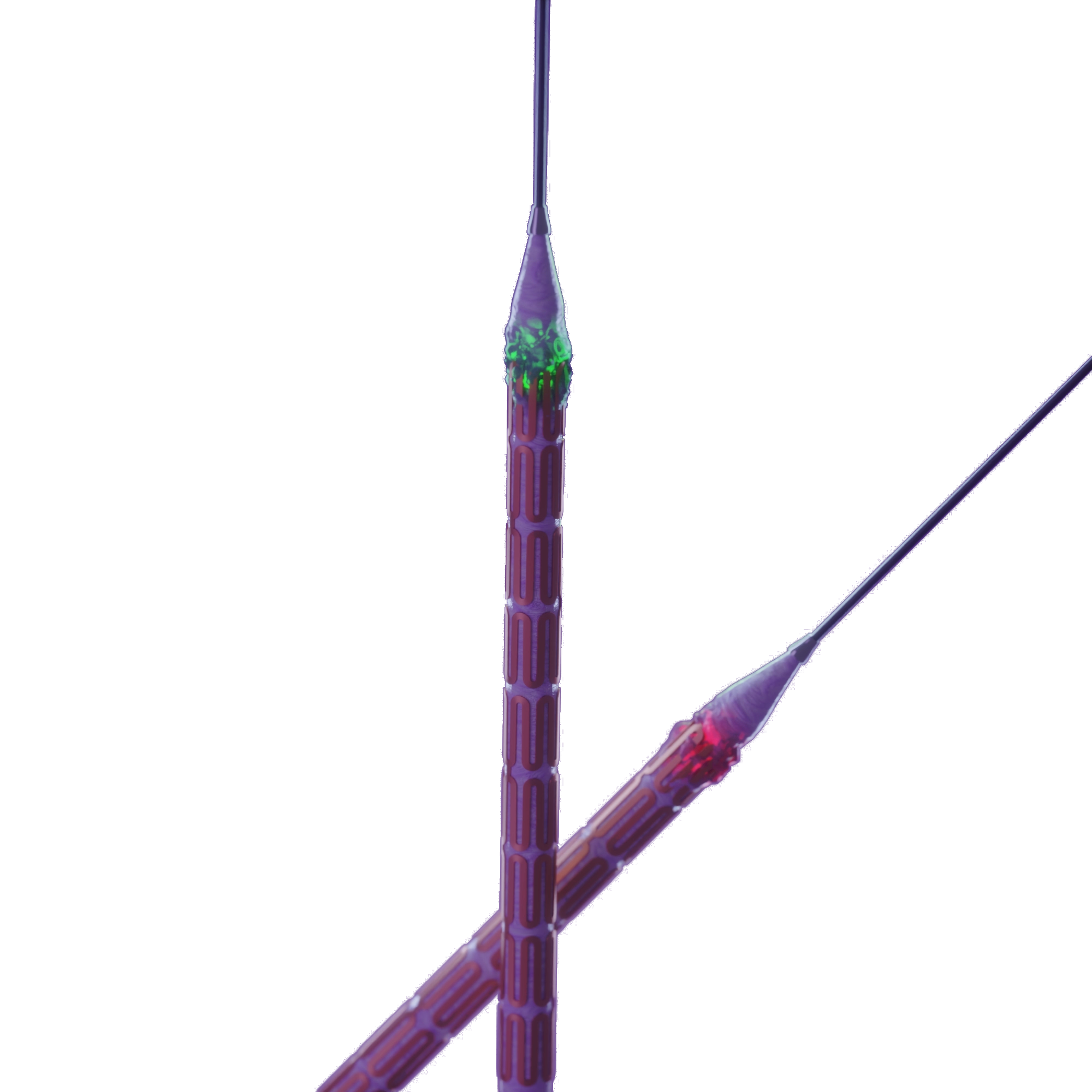
Edge Coating
The additional 0.5mm coating on the edge of the balloon which addresses the edge restenosis
05
Drug/Excipient
| Drug | Sirolimus |
| Drug Dose | 1.4µg/mm² |
| Drug Carrier | Customized biodegradable polymer matrix |
Stent
| Stent Material | L605 Cobalt Chromium Alloy |
| Stent Thickness | 73µm |
| Strut Width | 80µm(hinge)-120µm(strut) |
Delivery System
| Delivery System | RX/Monorail |
| Nominal Pressure | 8 Bar |
| Rated Burst Pressure | 14 Bar (Do not exceed RBP) |
| Guidewire Compatibility(max) | 0.014’’ |
| Guiding Catheter Compatibility | 5F |
| Crossing Profile | 0.038” (Reference diameter of 3.00mm) |
| Tip Entry Profile | 0.016’’ |
DRUG/EXCIPIENT
Drug
Drug Dose
Drug Carrier
1.27µg/mm²
Phospholipid
Catheter design
Rapid Exchange(RX) Design
| Stent Length (mm) | Stent diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2.25 | 2.50 | 2.75 | 3.00 | 3.50 | 4.00 | 4.50 | 5.00 | |
| 8 | EAN22508 | EAN25008 | EAN27508 | EAN30008 | EAN35008 | EAN40008 | EAN45008 | EAN50008 |
| 12 | EAN22512 | EAN25012 | EAN27512 | EAN30012 | EAN35012 | EAN40012 | EAN45012 | EAN50012 |
| 16 | EAN22516 | EAN25016 | EAN27516 | EAN30016 | EAN35016 | EAN40016 | EAN45016 | EAN50016 |
| 20 | EAN22520 | EAN25020 | EAN27520 | EAN30020 | EAN35020 | EAN40020 | EAN45020 | EAN50020 |
| 24 | EAN22524 | EAN25024 | EAN27524 | EAN30024 | EAN35024 | EAN40024 | – | – |
| 28 | EAN22528 | EAN25028 | EAN27528 | EAN30028 | EAN35028 | EAN40028 | – | – |
| 32 | EAN22532 | EAN25032 | EAN27532 | EAN30032 | EAN35032 | EAN40032 | – | – |
| 36 | EAN22536 | EAN25036 | EAN27536 | EAN30036 | EAN35036 | EAN40036 | – | – |
| 40 | EAN22540 | EAN25040 | EAN27540 | EAN30040 | EAN35040 | EAN40040 | – | – |
| 44 | – | EAN25044 | – | EAN30044 | EAN35044 | EAN40044 | – | – |
| 48 | – | EAN25048 | – | EAN30048 | EAN35048 | EAN40048 | – | – |
| 52 | – | EAN25052 | – | EAN30052 | EAN35052 | EAN40052 | – | – |

